6.6: 3d representation of orbitals Electron orbitals Orbitals cubic
Which are the orbitals(s,p,d,f) have center of symmetry? | Socratic
Orbitals chemistry electron atoms subshell order table atomic configurations periodic quantum number structure subshells electronic electrons energies which configuration energy Orbitals shapes draw shape 3d chemistry orbital five electron drawings four these 1.5-sublevels orbitals and electrons
Shapes of orbitals and their types
Sublevels (s, p, d, f)Five d -orbitals in a cubic crystal field which split into two e g Orbitals electron cubic orbitali orbitale 4p electrons spd atoms 4sWhy is the electron configuration of chromium "[ar]3d"^5"4s"^1" and not.
What is the electron configuration of chlorine?Electron levels electrons orbitals principle aufbau lower sublevel socratic configuration example 4s Electron configurations orbitals sublevel each has line orbital chemistry box withinS,p,d,f orbitals.

4s orbital electrons orbitals entering chemistry ell
Do electrons fill the lower energy levels first?What is the maximum number of electrons that can occupy the 3d orbitals Why do electrons enter the 4s orbital before entering the 3d orbital?Electron orbitals energy levels configuration fill configurations orbital order electrons sublevels electronic highest sub filled lowest map filling level increasing.
Orbitals sublevel shapes sublevels 2s 3s axis identical madeShapes of orbitals and sublevels Sublevel 4s socratic8.3 development of quantum theory – chem 1114 – introduction to chemistry.

Orbitals orbital quantum sublevels atomic explained spdf parsing bonding hybridization answer
Orbitals representation chemistry probability wave atomic wavefunction libretexts pageindex phaseOrbitals 3d representation chemistry chem libretexts electronic figure How many orbitals are in each sublevel? + exampleWhy is 4s orbital filled before 3d orbital?.
Spdf orbitals : parsing spdf orbital hybridization and simple bondingElectron orbital shells quantum atomic physics occurred Orbitals atomic atom sublevels structure shapes electron sub chemistry energy modern sublevel shape electrons elements level levels model atoms configurationsSublevel orbitals illustrate quantum nucleus.

7.6: 3d representation of orbitals
Orbitals sublevels electronsOrbitals orbitales orbital electrons electron cinco occupy lóbulos ncssm quantum subshell cuanticos 2.2: electron configurationsHow electrons fill orbitals and.
Chem orbitals shapes quantum chemistry model atoms theory orbital diagram electrons sublevels sublevel mechanics using wave axes spherical figure shapedOrbital orbitals subshell symmetry socratic Which are the orbitals(s,p,d,f) have center of symmetry?The sublevel.

How do you draw s,p,d,f orbitals?
Filling electrons order shell number maximum chemistry electron configuration each which electronic orbital 4s 3d filled why orbitals sublevels transitionOrbital orbitals 5f atomic quantum magnetic number electron 4f atom shapes subshell chemistry types structure difference many between together seven Electrons electron energy sublevels number level sublevel table orbital configuration chlorine each many periodic chart chem hold chemistry configurations does.
.


Which are the orbitals(s,p,d,f) have center of symmetry? | Socratic

Electron Orbitals | Definition, Subshells & Shapes - Lesson | Study.com

Shapes of Orbitals and their Types | Chemistry Skills
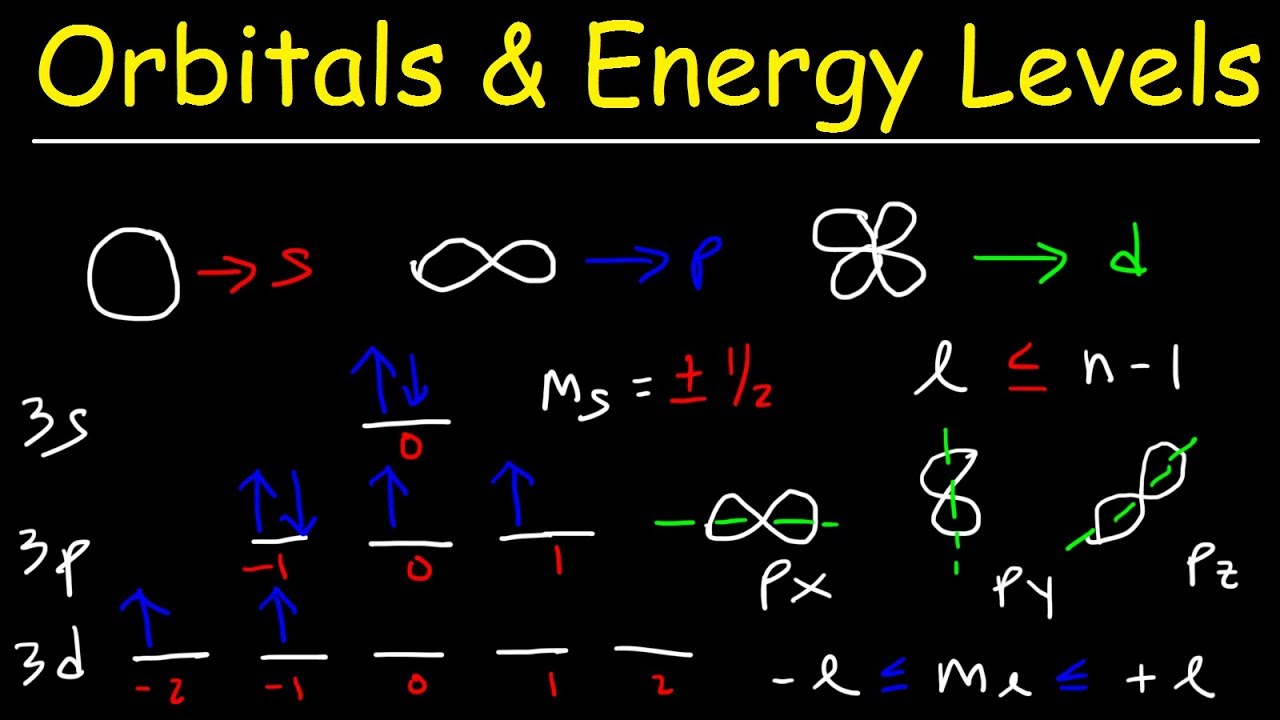
Spdf Orbitals : Parsing Spdf Orbital Hybridization And Simple Bonding
)/Std_Forms/how-ekect-fill-orbitals-sublevels_files/image006.jpg)
How Electrons fill Orbitals and
Do electrons fill the lower energy levels first? | Socratic
![Why is the electron configuration of chromium "[Ar]3d"^5"4s"^1" and not](https://i2.wp.com/useruploads.socratic.org/GzpxqflTSTm9KwkzEqYw_2-aufbau-principle.jpg)
Why is the electron configuration of chromium "[Ar]3d"^5"4s"^1" and not
